Filing an IND

Congratulations on hitting your company’s milestone! After years of work and maybe a few false starts, your team is ready to initiate a First-in-Human (FIH) Phase 1 clinical trial with its new drug candidate. What happens now? How does a Health Authority such as US FDA look at the data contained in your IND? What is the process? What can you expect to hear from them? Importantly, what content do they want in your IND, and how do they look at your data? These and other topics will be presented in our series on What to Expect After You File Your IND.
Click here for Part II: What is the FDA Looking For in Your IND?
Click here for Part III: How to Handle FDA Feedback on Your IND
Click here for Part IV: What Are the Grounds for Clinical Holds?
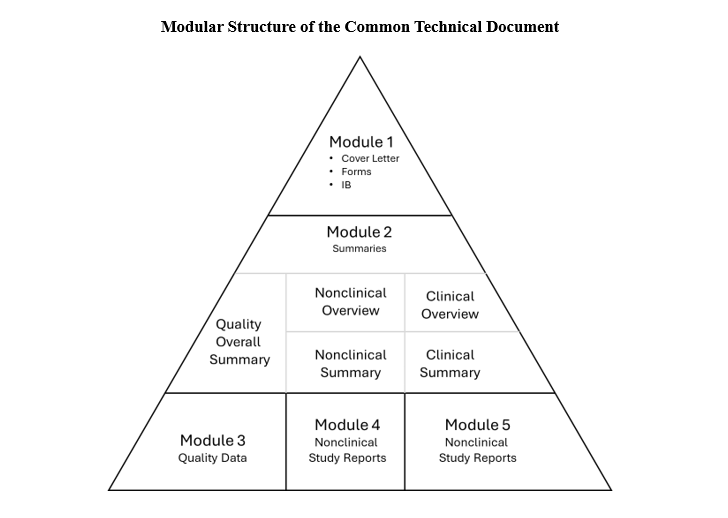
An IND contains a large number of documents that describe the properties and intended uses of the investigational agent, its chemical composition, biochemical and cellular effects, and its effects in animals. These documents are organized in a modular structure within the Common Technical Document (CTD Modules 1-5). The CTD is a standardized structure enabling the same application format to be used when submitting applications to any of the ICH regulatory authorities. The information and timelines in this article are specific to US FDA, or the Agency.
The FDA 30-day review period is an active period of communication between the Sponsor and the Agency. The first communication that the Sponsor is likely to receive is an acknowledgement, informing the Sponsor that the IND has been received. If there has been no pre-IND meeting, this will also be the point when the assigned IND number will be communicated. The acknowledgement will provide the date that FDA received the IND application. That date marks the initiation of the 30-day review period. The Sponsor will also receive the name and contact information for the individual who will be responsible for all communications between the Sponsor and the reviewing Division.
Typically, after an IND is filed, it takes up to a week for the project team at the FDA to be assembled. The key disciplines required for reviewing a new molecular entity (NME) IND are Clinical, Pharm/Tox, CMC, and ClinPharm, as well as their respective supervisors and both Division and Office leadership. Once the review team has been assembled, they begin their review of the data submitted to the IND. The core of the review takes approximately 2 weeks, during which time, the team collaborates on the review document. It’s common for the Sponsor to receive multiple Information Requests (IRs) from the FDA during the review period. Because the review clock is short, it is critical that the sponsor’s team is available to address questions in a timely fashion (often by EOB on the date of request). Akkeri’s experts have a wealth of experience responding to IRs and can help you clarify exactly what the Agency is asking for and formulate prompt, concise replies.
Each FDA review team member is responsible for reviewing the items pertinent to their discipline, and to complete the review according to the Division’s timeline. It is important that the modules are complete and that the necessary data are available, or the IND may be rejected on technical grounds. There are a number of guidance documents available that can guide Sponsors about structure and format of their IND, including information about how to file an electronic submission. A partial list of these guidance documents is provided below.
For more information about filing your IND, check out our other articles, or reach out to us at hello@akkeri.com.
Resources
IND Applications for Clinical Investigations: Regulatory and Administrative Components | FDA
Good Review Practices | GRPs | FDA
eCFR :: 21 CFR Part 58 — Good Laboratory Practice for Nonclinical Laboratory Studies
Guidance Documents
Electronic Regulatory Submission and Review | FDA
eCTD v4.0 Comprehensive Table of Contents Headings
Akkeri provides expert nonclinical drug development consulting services to support biotech and pharmaceutical companies in advancing therapeutic candidates to patients.
(737) 291-6009
hello@akkeri.com